Status and trends for harbor seals in the Salish Sea
Harbor seals were hunted from the 1870s to 1970s until they were protected in the United States by the 1972 Marine Mammal Protection Act and in Canada under the 1970 Marine Mammal Regulations in the Fisheries Act. The inland Washington harbor seal stock is estimated to be over 12,000, while the Strait of Georgia sustains approximately 39,000 harbor seals. Key threats include human disturbance, habitat degradation, loss of prey, and interaction with fishing gear and boats.

Overview
Harbor seals are the most commonly seen marine mammal in the Salish Sea and are the most abundant year round resident marine mammal species in the inland waters of Washington State (Gaydos and Pearson, 2011; Zier and Gaydos 2014). They are a generalist, opportunistic feeder, consuming over 60 different prey species. They will eat what is locally abundant so their diet composition can vary temporally, spatially and between individuals (Bromaghin et al. 2013,Thomas et al. 2022). Their only natural predator is the Bigg’s killer whale, which is increasingly active within the Salish Sea in recent years (Shields et al. 2018).
Harbor seals were hunted from the 1870s to 1970s for their pelts and bounty until they were protected in the United States by the 1972 Marine Mammal Protection Act (NOAA 2013a) and in Canada under the 1970 Marine Mammal Regulations in the Fisheries Act (Government of Canada 2013, DFO 2010). Since the mid-1990s, the Salish Sea population seems to have reached carrying capacity and has remained relatively stable (DFO 2010, Jeffries et al. 2003). The density of harbor seals in the Salish Sea is almost 3 harbor seals per square kilometer of ocean, possibly one of the most dense harbor seal populations in the world (Zier and Gaydos 2014).
Harbor seals generally remain relatively close and have high site fidelity to their haul out locations (DFO 2010, Hardee 2008). Thus, they are sensitive to human activities, including key threats such as human disturbance, habitat degradation, loss of prey, and interaction with fishing gear and boats.
Status, trends & events
Harbor seals have been plentiful in the waters of the Salish Sea, although historical (pre-exploitation) population size is unknown. They were hunted for bounty as well as their pelts, with over 500,000 killed from the 1870s until the 1970s on the coasts of British Columbia and WA state (BC: Olesiuk 2010, Bigg 1969, Fisher 1952; WA: Newby 1973, Scheffer and Slipp 1944). Both U.S. and Canadian harbor seal populations were protected in the 1970s, in the United States by the 1972 Marine Mammal Protection Act (NOAA 2013a) and in Canada under the 1970 Marine Mammal Regulations in the Fisheries Act (Government of Canada 2013, DFO 2010). From 1972 into the 1980s, harbor seal stocks grew exponentially at a rate of about 6% per year, reaching carrying capacity (around 50,000) in the 1990s and continue to be stable (Jeffries et al. 2003, see figure 1; DFO 2010; Majewski and Ellis 2022 - see fig 2)). The inland Washington harbor seal stock is estimated to be over 12,000 (Carretta et al. 2013), while the Strait of Georgia sustains approximately 39,000 harbor seals (Olesiuk 2010; Majewski and Ellise 2022). The Salish Sea covers 16,925 square kilometers of marine water (Gaydos et al. 2008), making the harbor seal density of almost 3 harbor seals per square kilometer of ocean possibly one of the most dense harbor seal populations in the world.
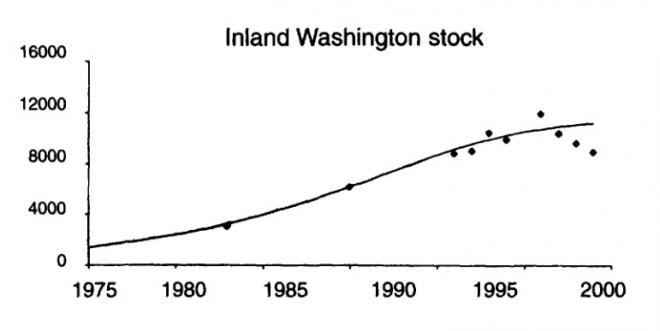
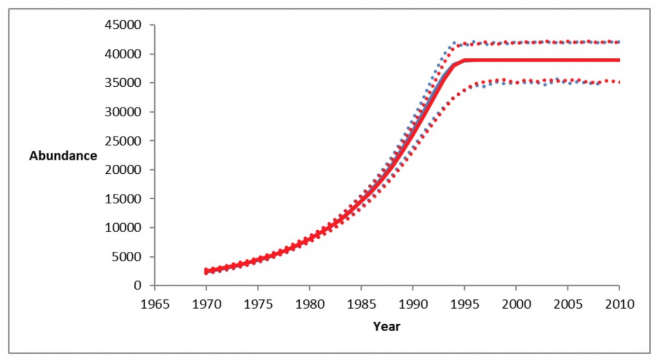
This increase in harbor seal numbers has been attributed, at least in part, to the increased occurrence of Transient, or Bigg’s killer whales in recent years. Bigg’s killer whales are the most significant predator of harbor seals in the Salish Sea (Ford et al. 2000; Scheffer and Slipp 1944). In BC waters they were the prey item in 52% of observed predation events by Bigg’s killer whales in BC waters (Ford et al. 2013). It has been estimated that Bigg’s killer whales could be eating more than 2% of the total harbor seal population per year (Shields et al. 2018).
Natural history
Distribution and occurrence
The harbor seal is the most commonly seen marine mammal in the Salish Sea and can be found throughout the region year round (Gaydos and Pearson, 2011). The Washington Inland Waters Stock (Salish Sea) is made up of 3 distinct populations based on studies of pupping phenology, mitochondrial DNA, and microsatellite variation: 1) Southern Puget Sound (south of the Tacoma Narrows Bridge); 2) Washington Northern Inland Waters (including Puget Sound north of the Tacoma Narrows Bridge, the San Juan Islands, and the Strait of Juan de Fuca); 3) Hood Canal (Huber et al. 2012; Carretta et al. 2013, see Figure 3). Although some male harbor seals travel between the Salish Sea and Washington’s outer coast (Peterson et al. 2012), in general harbor seals in the Salish Sea are genetically distinct from those found on the outer coast (Lamont 1996).

Harbor seals are often seen in water and also on land when they “haul-out”, or come out of the water to rest on land. They use well over 1900 haul-out locations throughout the region (1,400 in BC, 507 in WA) that include sand bars, mudflats, tideflats, rocks, reefs, ledges, all types of beaches, islands, logbooms, docks, and floats (DFO, 2010; Olesiuk 2010; Jefferies et al. 2000). They can utilize estuarine habitats (Luxa and Acevedo-Gutierrez 2013) as well as freshwater, where they have been documented traveling up to 500km upriver (DFO 2010). They are non-migratory, typically moving and foraging within 30 kilometers of primary haul-out sites in the Salish Sea (Peterson et al. 2012, DFO 2010). However, males have been documented to travel much farther than females. In one study using satellite tags, females remained within 41.6km from the capture site, whereas some males traveled over 100km at least once (Peterson et al. 2012). Additionally, there is a high degree of individual variation in home range in adult harbor seals.

Photo: Cindy R. Elliser/Pacific Mammal Research
What eats them?
The only known predator of harbor seals in the Salish Sea are Bigg’s killer whales (Ford et al. 2000, Scheffer and Slipp 1944), which have been increasing in recent years (Shields et al. 2018). Observations have shown that all regularly encountered groups of Bigg’s killer whales predate upon both pinnipeds and cetaceans (Ford et al. 1998). Sightings of these whales near shore and haul-out locations peak during the harbor seal pupping season due to increased prey availability (Baird and Dill 1996). They may also kill harbor seals for purposes other than consumption, perhaps as surplus killing or play behavior (Gaydos et al. 2005).
Outside of the Salish Sea, sharks are a significant predator of harbor seals (Scheffer and Slipp 1944), including great white sharks (Carcharodon carcharias: Anderson et al. 2008, Scheffer and Slipp 1944) and Pacific sleeper sharks (Somniosus pacificus: Taggart et al. 2005). Great white sharks have not officially been documented in the Salish Sea, but they are common along the coast of Washington and could come into the area occasionally. Steller sea lions (Eumetopias jubatus) have emerged as a predator of harbor seals in Alaska (Mathews and Adkison 2010). Salish Sea Steller sea lions are not known to prey on harbor seals. However, it is possible a predation event could occur as the two species’ ranges overlap in the area.
As mentioned previously harbor seal abundance was reduced by humans drastically in British Columbia and Washington State from the 1870s until the 1970s. They were hunted commercially for their pelts and culls were instituted as predator control and to protect commercial and sports fishermen (Huber and Laake 2002, Olesiuk 2010, NOAA 2003, Newby 1973) until they were federally protected in the early 1970s. It is unknown what effects the culling programs had on fisheries and the ecosystem at large (Bowen and Lidgard 2013).
What do they eat?
Salish Sea harbor seals are generalist and opportunistic predators feeding on at least 60 different species of fish as well as several species of crustaceans and mollusks. Salmonids (Oncorhynchus spp.), Pacific Herring (Clupea pallasii), Pacific Sand Lance (Ammodytes hexapterus), Northern Anchovy (Engraulis mordax), Walleye Pollock (Theragra chalcogramma), Shiner Perch (Cymatogaster aggregata), were found to be significant proportions of their diet in the San Juan Islands and nearby estuarine ecosystems (Lance et al., 2012), with salmonids and herring (Clupeidae) being the two most energetically important prey groups for biomass consumed by harbor seals in the San Juan Islands (Howard et al. 2013). In a larger transboundary study, Pacific Hake and Pacific Herring were found to occur the most, comprising 46% of the identified prey, with salmon species present, but substantially less proportionally (1.6% - 6%, Thomas et al. 2022). In one Salish Sea estuarine location the remains of small, possibly juvenile, mammals were documented and thought to be the first study to identify mammals as harbor seal prey (Luxa and Acevedo-Gutiérrez 2013). More rare prey species have been documented including a harlequin duck (Histrionicus histrionicus, Tallman and Sullivan 2004), neried worms (Luxa and Acevedo-Gutiérrez, 2013) and spotted ratfish (Hydrolagus colliei, Akmajian et al. 2012), with the latter ultimately killing the harbor seals when the poisonous dorsal spines of the fish perforated their esophagus or stomach.

Due to their opportunistic nature, harbor seals will adjust their foraging behavior in relation to prey availability and often varies between seasons (e.g. Thomas et al. 2011). Many studies have shown that diet can also vary with diel patterns, between males and females, between breeding and non-breeding individuals and haul-out locations. There is considerable variation in the dive profiles of individual seals even between relatively close (~20km apart) haul out sites, showing that foraging behavior can be site dependent (Wilson et al. 2014). This suggests that there is prey specialization or habitat exploitation by different haul-out site groups (Wilson et al. 2014). Prey specialization and variability has been documented in other locations in the Salish Sea as well (London et al. 2001, Lance et al. 2012, Bromaghin et al. 2013, Thomas et al. 2022). Due to their opportunistic nature harbor seals can take advantage of locally abundant prey, creating large variations in diet and foraging behavior across time and space. Studies like these emphasize the importance of examining harbor seal behavioral variation on a small spatial scale (Wilson et al. 2014) to better understand their foraging ecology and impact on various fish stocks, particularly those that are threatened, endangered or depleted.
Concern has been raised over the impact of harbor seals (and other pinnipeds) and if they may cause or exacerbate fisheries declines or hinder the recovery of depleted stocks (Ward et al. 2012). The predatory role of harbor seals in the Strait of Georgia ecosystem on fish including salmon, herring and hake is of ongoing interest (Olesiuk et al. 1990, Cottrell 1995, Li et al. 2010, Priekshot et al. 2013). They consume at least 14 of 31 fish species in the Salish Sea that are listed as threatened, endangered or a candidate for listing (Gaydos and Brown 2011, Zier and Gaydos 2014). Salmon stocks are of primary concern, especially Chinook, because that is the primary prey species for the struggling Southern Resident Killer Whales. As indicated above, salmon are a common prey species in harbor seal diet studies, which has raised concerns over their impact on salmon recovery (Nelson et al. 2018, Thomas et al. 2017). However, until recently, limitations in methodology have prevented the accurate quantification of the proportion of salmon in their diet (Thomas et al. 2022), which is required to fully understand their impact on salmon populations. Using new DNA metabarcoding, Thomas et al. (2022) found that for salmon species, on average, harbor seal diets contained: 6% chum, 3.5% chinook, 2.5% pink, 2.2% sockeye, and 1.6% coho salmon, however there was considerable diet variability between haul-out sites and regions in the Salish Sea (samples were from throughout Canadian and United States waters). The data presented in that study are publicly available to help facilitate an open exchange of ideas concerning harbor seal trophic ecology in the Salish Sea (Thomas et al. 2022).
Biology
Harbor seals are the second smallest phocid in the world (Smith et al. 1990). The average lifespan for harbor seals is 8 (males) to 10 (females), though they have been known to survive as long as 20 (male) to 30 (female) years (DFO 2010, Osborne et al. 1998, Bigg 1969).
Their coloration patterns vary from pale white to nearly black coats with light or dark spots, rings and splotches (DFO 2010, Jeffries et al. 2000, Scheffer and Slipp 1944) and can be used to photo-identify individuals. Every year after mating season in mid to late summer, harbor seals molt, or shed their pelage (Daniel et al. 2003, Walker 1999, Ling 1972, Stutz 1967). Harbor seals tend to increase how much they haul out during the molting season (Patterson and Acevedo-Gutiérrez 2008, Harris et al. 2003). This may be because they require less food, or because blood flow to the skin increases during molting and so resting on land reduces heat loss compared to trying to maintain body temperature in cold waters (Watts 1996).

Harbor seals haul-out for molting, but also for reproduction, rest and predator avoidance. The numbers of harbor seals that haul-out usually peaks in summer (corresponding to molting and breeding, Patterson and Acevedo-Gutiérrez 2008, Harris et al. 2003), and diel patterns peaking around midday or at afternoon and evening low tides (Cowles et al. 2013, Cunningham et al. 2009, Patterson and Acevedo-Gutiérrez 2008, Simpkins et al. 2003, Watts 1996, Thompson et al. 1989) but can vary between locations in the Salish Sea. Factors like time of day, season, tide level, weather, and human disturbances are known to affect haul-out patterns (London et al. 2012, Acevedo-Gutiérrez and Zarelli 2011, Patterson and Acevedo-Gutiérrez 2008, Härkönen et al. 1999, Watts 1996, Thompson et al. 1989). How many seals are hauled-out at a given time varies between the sexes, among age groups and depends on environmental factors.
Although in general haul-out site fidelity is high (DFO 2010, Hardee 2008), it can vary greatly. In the San Juan Islands the majority of harbor seals had a haul-out fidelity of 75% or greater (Suryan and Harvey 1998). In the wider Salish Sea, male seals at rocky reef sites had low haul-out site fidelity and were distributed along many haul-out regions up to 120km apart (Hardee 2008). At soft sediment sites in Padilla Bay seals of both sexes remained within 10 km of the bay, and half of the seals had 100% haul-out site fidelity (Hardee 2008). Thus as with foraging differences, specific haul-out behavior may differ within and between harbor seal populations, haul-out locations, as well as among individual harbor seals.
Eyes: Harbor seals have many adaptations for seeing in air and underwater. They have multifocal lenses and on land they can see as well as other terrestrial mammals. They can detect objects as effectively as a cat due to high contrast sensitivity (Hanke et al. 2009). They also have vertical and horizontal optokinetic nystagumuses that allow their eyes to track movement well in all planes, aiding in their ability to maneuver in the 3-D underwater environment (Hanke et al. 2009). Like cats, wolves and dogs, but unlike other pinniped species, harbor seal retina have a visual streak and an area centralis which gives good peripheral detection of movement. Harbor seals can adapt rapidly to changes in light which is necessary when diving from daylight to depths where light is minimal. This ability is due to their cornea and pupil structure and tapetum cellulosum that helps them see at night (Hanke et al. 2009). Their eyes are situated dorsal which gives them a wide field of vision, though ventrally this is limited. Thus harbor seals will often swim upside to do look below them while foraging (Hanke et al. 2009, Kilian et al., 2015)
Whiskers (vibrissae): Adult harbor seals have around 42 whiskers or vibrissae (Scheffer and Slipp 1944). The whiskers are undulated (wavey) which reduces water flow resistance and allows the seals to keep them abducted (not flattened against their snout) while they swim (Hanke et al. 2010). The whiskers are very sensitive and can detect objects by touch and are critical for following hydrodynamic trails - tracking water disturbances over a greater distance than can be done with sight or hearing (Hanke et al. 2010).
Diving: Harbor seals can dive as deep as 90 meters and for around 6 minutes (Wilson et al. 2014). Their adaptations for diving include tolerating carbon dioxide and lactic acid build up in the blood, manually inducing a reduction in heart rate (bradycardia) to conserve oxygen and reducing blood circulation to peripheral blood vessels to conserve oxygen from the brain and heart (Walker 1999). It has been shown that blood redistribution in seals is under some degree of cognitive control and likely used to help modulate reoxygenation of the brain (McKnight et al. 2019).
Reproduction: Unlike many pinniped species, harbor seals do not mate or guard territories (or harems) on land. Instead males will perform displays that include slapping their posterior flippers on the surface of the water, rolling, growling (Sullivan, 1981, Zier and Gaydos 2014) or performing underwater displays (Van Parijs et al. 1997) that are thought to attract females. The female may assess the male’s fitness and then instigate an aquatic encounter. Copulation is believed to occur underwater (Sullivan 1981,Thompson et al. 1994). Males may mob a female and attempt to mount her in the water column for copulation (Allen, 1985). Males become sexually mature between 3 and 6, and females between 2 to 5 (Bigg 1969a). Mating occurs after weaning (Scheffer & Slipp, 1944).
After fertilization, the implantation and development of the blastocyst is delayed about 2.5 months in what is termed embryonic diapause, or delayed implantation (Temte 1985, Bigg 1969), which is a common trait among pinniped species. Females give birth to one pup per year (Hayes et al. 2006). Pupping in the Salish Sea occurs from June–October (Figure 2, Seekins, 2009) and varies with latitude. Somewhat counterintuitively, later pupping times are found in the more southern areas of the Salish Sea compared to the northern areas, where seal pups are born an average of 88 days later than those on the Washington coast at similar latitudes (Seekings 2009, see Figure 4; Temte 1985). Usually harbor seal pups will molt their lanugo (white fetal hair) in-utero, however roughly 20% of pups are born with lanugo and are generally smaller than other pups (Cottrell et al. 2002).
![Pupping time frames by location in the Salish Sea Map showing harbor seal pupping time frames by location in the Salish Sea.]](/sites/default/files/styles/bodywidth_2-col_660px/public/topical_articles/images/PuppingTimeframeWA_1024.jpg?itok=C7NkWTSc)
Pups are precocious and active after birth (DFO 2010, Jeffries et al. 2000) and are nursed for about 30 days (Cottrell et al., 2002). It is important to note that harbor seal mothers will leave their pups unattended during this nursing period in order to forage, and the pups are likely unharmed. People should observe from afar, at a distance of at least 100 yards, for signs of injury or the return of the mother, but otherwise these pups should be left alone (DFO 2006, NOAA 2013b). Although pups can swim soon after birth, their muscles are not fully developed until well after weaning (Prewitt et al. 2010) and they can be seen riding on their mothers’ backs during their first few weeks of life (Lawson and Renouf 1985). After weaning, pups will fast 14 to 17 days and lose 21% mass by 5 weeks (Muelbert and Bowen 1993).

Pups are thought to learn foraging behaviors during their first 32 days of life (pre-weaning while they are still with their mother, Gaydos et al. 2012). They will forage near their primary haul-out site during their 3 to 6 week nursing period, the length of which varies by location (Cottrell et al. 2002, Stein 1989, Bigg 1969a, Scheffer and Slipp 1944). A study comparing rehabilitated pups to wild pups showed that rehabilitated pups traveled nearly 3 times farther daily and dispersed over 3 times farther from the release site, suggesting that wild seals imprint on foraging areas during their first month of life with their mothers (which the rehabilitated seals do not have) (Gaydos et al. 2012). However limited data from outside the Salish Sea showed different patterns, suggesting that location specific factors could influence the behavior of wild and rehabilitated seal pups (Gaydos et al. 2012).
In water behavior: Little is known about the behavior of harbor seals in the water compared to when they are hauled-out (Boness 1999). Other pinniped species in the otariid family (sea lions and fur seals), frequently gather together in large groups in the water, a behavior known as rafting, however this is not known for phocid species (seals). Harbor seals will sometimes congregate in the water on specific occasions when swimming near a haul-out (Scheffer and Slip 1944) and in areas of concentrated prey and restricted space like forced bottlenecking at channels (Zamon 2001) or mouths of rivers (Marston et al. 2002), but otherwise are thought to be solitary at sea (Bonness 1999). However, recently in the Salish Sea harbor seals have been observed congregating together (in groups of 6 to more than 150, with animals 1-2 body lengths from each other), a behavior previously undocumented in the literature for this species (Elliser et al. 2022). Most of the observations were obviously foraging behavior, but others appeared to be resting, traveling or socializing (Elliser et al. 2022). Mating behavior (male display) was not observed in any grouping, and although there are safety in numbers, no known harbor seal predators were observed in the vicinity of any grouping (Elliser et al. 2022). It is likely that these events are primarily driven by foraging needs, but more research is needed to fully understand this behavior (Elliser et al. 2022).

Photo: Cindy R. Elliser/Pacific Mammal Research
Threats
Predator avoidance: Hauling-out is generally a good strategy to avoid aquatic predators like killer whales. In Hood Canal, the probability of a seal hauling-out increased by 40-50% during high presence of Bigg’s killer whales in the area in 2003 and 2005 (London et al. 2012). While hauled out, seals remain vigilant of potential threats (which can include eagles, turkey vultures, Grizzly and Black bears, wolves, domestic dogs, coyotes, killer whales that may use tactics to knock them off the haul-out, etc.) and if a potential threat is spotted, the seals may flush into the water. This can also occur due to human disturbance and has been noted for many locations in the Salish Sea (Johnson and Acevedo-Gutiérrez 2007, Suryan and Harvey 1999). This causes the seals to expend excess energy, and lose resting time, which can be detrimental to long-term health. It has also been documented that seals can become habituated to high levels of disturbance and in turn exhibit less anti-predatory response. Habituated seals save energy by not flushing into the water, for example from usually harmless boat traffic, however this reduced sensitivity increases the risk of natural predation as they are less vigilant (Olson 2013).
Mortality other than predation: Harbor seals can be infected with a variety of infectious diseases, some of which are zoonotic and can be transmitted to humans, domestic animals or other wild species (see review in Zier and Gaydos 2014).
Stranding records show age-related seasonal patterns, with pups found most often in summer, weaned pups during fall and adults and subadults in summer and fall (Ashley et al. 2020). 70% of pups died from nutritional causes like emaciation, likely connected to the health of the mother during pregnancy, behavior of mother post-partum (mismothering), or maternal separation possibly caused by human disturbance (Ashley et al. 2020). There may be an interaction between poor nutritional condition and enhanced susceptibility to infectious diseases (Ashley et al. 2020). Subadults and adults primarily presented with gross lesions and infectious disease (42%) and non-anthropogenic trauma (27%) (Ashley et al. 2020). Primary causes of mortality were related to nutrition, infection, non-anthropogenic and anthropogenic trauma, with additional causes of death relating to congenital disorders, predation, human interaction and infections, including zoonotic and multi-drug resistant pathogens (Ashley et al. 2020). A study including 74 dead-stranded harbor seals in the Salish Sea found 35% had bacteria resistant to at least one antibiotic, and 24% had bacteria that were multi-drug resistant (Norman et al. 2021). This may reflect a large environmental reservoir of antibiotic resistant organisms in the Salish Sea that can have impacts on other species and humans as well (Norman et al. 2021). Congenital disorders (the most common being cleft palate, cleft lips, and cardiac defects) at a rate of 2.9% is the endemic level of congenital disease in this stable Salish Sea population (D’Agnese et al. 2021).
Harbor seals can also be injured by boats, which may be an increasing issue with more boats on the water, and a healthy harbor seal population at carrying capacity. A retrospective study (2002-2019) on stranded harbor seals in the Salish Sea found 27 cases of fatal propeller strikes, with 64% being weaned pups (Olson et al. 2021). The number of strikes significantly increased over the study period, indicating increased interactions between boats and seals (Olson et al. 2021).
Toxins: Human activity has been polluting the Salish Sea since at least the 1800s including lead, mercury, silver, copper, hydrocarbons, persistent organic pollutants (POPs, such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichlorethane (DDT), Lefkovitz et al. 1997) and polybrominated diphenyl ethers (PBDEs) that replaced PCBs but are also toxic. PDBs and DDT were banned in the US and Canada in the 1970s, and PBDEs in Washington State in 2008 and 20011 due to their toxicity (Ross 2006). Large exposure to hydrocarbons can cause direct mortality (e.g. after a large oil spill like the Exxon Valdez, Hoover-Miller et al. 2001). However, there are also long lasting effects. Many of these chemicals are lipophilic (dissolve in fat) and do not break down quickly, meaning they bioaccumulate in organisms and top predators like harbor seals will have higher contaminant loads (Tabuchi et al. 2006). The level of contamination varies with location in the Salish Sea, which relates to some seals eating from more highly contaminated fish stocks (Ross et al. 2013).
PCBs can compromise immune system health. Harbor seals in the Salish Sea with higher concentrations of PCBs had reduced the immune responses. These effects can reduce the seal’s ability to defend against pathogens (Zier and Gaydos 2014). PCBs can also affect growth and development by interfering with thyroid hormones and gene expression, which can alter the structure and function of blubber, which is of primary importance for energy storage, insulation, buoyancy control and nutrient storage (Tabuchi et al. 2006). Though concentrations of legacy contaminants do seem to be dropping in Salish Sea harbor seals (e.g. legacy PCB concentration dropped 81% from 1984 to 2003 (Ross et al. 2013).
Rehab: Although harbor seals are at or near carrying capacity (Jeffries et al. 2003), stranded harbor seal pups are rehabilitated with strong public support (Gaydos 2012). NOAA authorizes several organizations, like Wolf Hollow Wildlife Rehabilitation Center on San Juan Island, to rehabilitate seals and hotlines are maintained in WA state and BC where the public can report strandings (NOAA 2013b). However, prior to 2021 rehabilitation of harbor seals has been limited to pups. In spring of 2021, Sealife Response Rehabilitation and Research (SR3) built the only hospital in the Pacific Northwest (US) that is dedicated to marine wildlife. Previously there was no permitted facility capable of caring for, evaluating, or rehabilitating endangered marine mammals, or with the ability to hold adult seals, sea lions, sea otters, or harbor porpoises. In BC, the Vancouver Aquarium runs Canada’s only dedicated marine mammal rescue facility and rehabilitates seals, sea lions, sea otters, sea turtles and small cetaceans (DFO 2006).
Determining if a rehabilitated harbor seal can be released is done through detailed analysis of historical, developmental, behavioral, ecological, and medical criteria to ensure that the seal is capable of surviving in the wild and will not be a threat to other organisms. The seals must be able to hunt and feed for themselves, swim and dive effectively and pass a health test conducted by a veterinarian (Zier and Gaydos 2014). The release site is often a haul-out near the stranding site, so the seal may rejoin its genetic stock and natural home range. The seals are flipper tagged so they can easily be identified after release, and long-term data on survivability may be obtained (Zier and Gaydos 2014). The general protocols for harbor seal rehabilitation are largely the same in BC (Vancouver Aquarium 2013) and the US (National Marine Fisheries Service, U.S. Fish and Wildlife Service).
Current policy issues
This most prominent policy issue relating to harbor seals is their effect on depleted salmon populations. Over the last few years there have been increasing pressure to actively manage pinniped populations (i.e. cull or lethal removal of individuals). Although pinniped predation is a plausible explanation for the lower abundance of salmon in WA, the evidence does not support a definitive conclusion that they are the primary cause (Washington State Academy of Sciences 2022). The scientific evidence required to justify a cull of a marine mammal species is usually highly uncertain (Bowen and Lidgard 2012). Indeed there continue to be uncertainties around whether pinniped predation adds to salmon mortality or they are killing salmon that would otherwise die before adulthood, what the role of other prey (like herring) in either increasing pinniped populations that feed on salmon or decreasing predation by being an alternative food source, and whether the indirect effect of pinniped predation on salmon predators (like Pacific hake) offsets the direct impact of predation on salmon (Washington State Academy of Sciences 2022). In addition there is considerable variation in harbor seal diet between individuals, age, sex and haul-out locations as summarized previously. Thus there may be certain individuals or locations that have a larger impact on salmon populations, and reducing numbers across the board may not produce the intended outcomes.
It has been suggested that experimentally changing pinniped populations (in a more targeted approach) may be needed at spatial and temporal scales that would make a meaningful impact to the ecosystem to understand whether or not they are impeding the recovery of salmon (Washington Academy of Sciences 2022). Although in the past marine mammal culling programs can be very effective at reducing predator density, they often have to include a large proportion of the population (>50%), have rarely had measurable objectives (in relation to prey populations) and their success has not been evaluated (Bowen and Lidgard 2013). In addition, they often have non-intuitive and unintended consequences for the target species and other prey and predators in the ecosystem (Bowen and Lidgard 2013). In complex ecosystems like the Salish Sea it can be difficult to understand the impacts of removing a large proportion of a species’ population (e.g. the uncertainties listed above). Bigg’s killer whales also have a population controlling effect, eating substantial amounts of harbor seals each year (at least 2% per year), which should be considered when evaluating management actions (Shield et al. 2018).
A non-lethal option, like sterilization of adult females, has been used for many terrestrial animals and proposed as another option for management of harbor seal populations (by itself or in combination with lethal measures, Nelson et al. 2023). Another consideration is looking at the fate of hatchery fish. Predators of juvenile salmon (fish, birds and marine mammals including harbor seals) may be choosing prey based on size. Research indicates that current hatchery practices release Chinook salmon in the size range preferred by these predators (Nelson et al. 2019). Changing hatchery practices could increase the amount of hatchery salmon that may reach adulthood.
More research on the diet, behavior and ecology of harbor seals and their prey is needed, along with investigation into other management options, to determine if any type of active management is required and/or if other options (or a combination of these) can culminate in the proposed outcomes of reduced predation on and recovery of salmon populations. Any program that suggests lethal removal should be based on scientific analysis with stated and measurable objectives to be evaluated during follow up monitoring (Bowen and Lidgard 2013).
Data sources & gaps
References
Acevedo-Gutiérrez, A., and S. Cendejas-Zarelli. 2011. Nocturnal Haul-Out Patterns of Harbor Seals (Phoca vitulina) Related to Airborne Noise Levels in Bellingham, Washington, USA. Aquatic Mammals 37(2): 167-174. DOI: 10.1578/AM.37.2.2011.167.
Akmajian, A. M., D. M. Lambourn, M. M. Lance, S. Raverty, and J. K. Gaydos. 2012. Mortality Related to Spotted Ratfish (Hydrolagus colliei) in Pacific Harbor Seals (Phoca vitulina) in Washington State. Journal of Wildlife Diseases 48(4): 1057-1062. DOI: 10.7589/2011-12-348.
Allen, S.G. 1985. Mating behavior in the harbor seal. Mar. Mamm. Sci. 1: 84-87.
Anderson, S. D., B. H. Becker, and S. G. Allen. 2008. Observations and Prey of White Sharks, Carcharodon carcharias, at Point Reyes National Seashore: 1982-2004. California Fish and Game 94(1): 33-43.
Ashley, E.A., Olson, J.K., Adler, T.E., Raverty, S., Anderson, E.M., Jeffries, S. and Gaydos, J.K. 2020. Causes of Mortality in a harbor seal (Phoca vitulina)) Population at Equilibrium. Frontiers in Marine Science 7, https://doi.org/10.3389/fmars.2020.00319
Baird, R. W., and L. M. Dill. 1996. Ecological and Social Determinant of Group Size in Transient Killer Whales. Behavioral Ecology 7(4): 408-416.
Bigg, M. A. 1969. The Harbour Seal in British Columbia. Bulletin of Fisheries Research Board No. 172. 33pp.
Boness, D.J. 1999. Chapter 8 Behavior, pinnipeds. In: Biology of marine mammals
(Reynolds III, J.E. & Rommel, S.S., eds). Smithsonian Institution Press,Washington, DC, p. 326-347
Bowen, W. D., and D. Lidgard. 2013. Marine Mammal Culling Programs: Review of Effects on Predator and Prey Populations. Mammal Review 43: 207- 220. DOI: 10.1111/j.1365-2907.2012.00217.x.
Bromaghin, J. F., M. M. Lance, E. W. Elliott, S. J. Jeffries, A. Acevedo-Gutiérrez, and J. M. Kennish. 2013. New Insights into the Diets of Harbor Seals (Phoca vitulina) in the Salish Sea Revealed by Analysis of Fatty Acid Signatures. Fishery Bulletin 111(1): 13-26. DOI: 10.7755/FB.111.1.2.
Carretta, J. V., E. Oleson, D. W. Weller, A. R. Lang, K. Forney, J. Baker, B. Hanson, K. Martien, M. M. Muto, M. S. Lowry, J. Barlow, D. Lynch, L. Carswell, R. L. Brownell Jr., D. K. Mattila, and M. C. Hill. 2013. U.S. Pacific Marine Mammal Stock Assessments: 2012. NOAA-TM-NMFS- SWFSC-504. NOAA SWFSC, La Jolla CA. 384 pp. Available at: https://repository.library.noaa.gov/view/noaa/4464
Cottrell, P. E., S. Jeffries, B. Beck, and P. S. Ross. 2002. Growth and Development in Free-Ranging harbor Seal (Phoca vitulina) Pups from Southern British Columbia, Canada. Marine Mammal Science 18(3): 721- 733.
Cottrell, P. E. 1995. Diet, activity budgets, and movement patterns of Harbour Seals (Phoca vitulina) in Cowichan Bay and adjacent areas. M.S. Thesis, University of Victoria, British Columbia. 118p. 1995.
Cowles, J. D., S. M. Henson, J. L. Hayward, and M. W. Chacko. 2013. A Method for Predicting Harbor Seal (Phoca vitulina)) Haulout and Monitoring Long- Term Population Trends Without Telemetry. Natural Resource Modeling 0(0): xxxx.
Cunningham, L., J. M. Baxter, I. L. Boyd, C. D. Duck, M. Lonergan, S. E. Moss, and B. McConnell. 2009. Harbour Seal Movements and Haul-Out Patterns: Implications for Monitoring and Management. Aquatic Conservation: Marine and Freshwater Ecosystems 19: 398-407. DOI: 10.1002/aqc.938
D'Agnese, E.R., Lambourn, D.M., Olson, J.K., Huggins, J.L., Raverty, S., Garner, M.M., Calambokidis, J., Scott, A.A., Jeffries, S.J., Gaydos, J.K. 2021. Congenital Diseases in Harbor Seals (Phoca vitulina richardsii) from the Salish Sea. J Wildl Dis. 57(3):672-677. doi: 10.7589/JWD-D-20-00179. PMID: 34015807.
Daniel, R. G., L. A. Jemison, G. W. Pendleton, and S. M. Crowley. 2003. Molting Phenology of Harbor Seals on Tugidak Island, Alaska. Marine Mammal Science 19(1): 128-140.
DFO. 2006. Marine Mammal & Sea Turtle Reference Manual: A Guide to Sightings, Strandings (Incidents), Legislation, Licensing and Species Identification. DFO. Pacific Region Marine Mammal Team.
DFO. 2010. Population Assessment Pacific Harbour Seal (Phoca vitulina richardsii). DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2009/011.
Elliser, C.R., Anderson, D.A., Derie, T., MacIver, K., and Shuster, L. 2022. Open water grouping behaviour of harbour seals (Phoca vitulina richardii) of the Salish Sea. Behaviour 159: 1375-1386.
Fisher, H. D. 1952. The Status of the Harbour Seal in British Columbia, with Particular Reference to the Skeena River. University of Toronto Press for the Fisheries Research Board of Canada. Ottawa. 58 pp.
Ford, J.K.B, E.H. Stredulinsky, J.R. Towers and G.M. Ellis. 2013. Information in Support of the Identification of Critical Habitat for Transient Killer Whales (Orcinus orca) off the West Coast of Canada. DFO Canadian Science Advisory Secretariat Research Document 2012/155. iv + 46 p.
Ford, J. K. B., G. M. Ellis, and K. C. Balcomb. 2000. Killer Whales, 2nd Ed. University of Washington Press. Seattle, Washington. 104 pp.
Ford, J.K.B, G.M. Ellis, Barrett-Lennard, L.G., Morton, A.B., Palm, R.S. and Balomb III, K.C. 1998. Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Canadian Journal of Zoology 76:1456-1471.
Gaydos, J. K. 2012. Survivor, Seal Style: Post-Rehabilitation Research. Proceedings of the 2012 North American Veterinary Conference, Orlando, Florida, January 2012. 2 pp.
Gaydos, J. K., L. I. Vilchis, M. Lance, S. J. Jeffries, V. Greenwood, P. Harner, and M. H. Ziccardi. 2012. Postrelease Movement of Rehabilitated Harbor Seal (Phoca vitulina richardii) Pups Compared with Cohort-matched Wild Seal Pups. Marine Mammal Science. DOI: 10.1111/mms.12002.
Gaydos, J. K., and N. A. Brown. 2011. Species of Concern within the Salish Sea: changes from 2002 to 2011. Proceedings of the 2011 Salish Sea Ecosystem Conference, Vancouver, BC, October 25-27, 2011. 12 pp.
Gaydos, J. K., and S. Pearson. 2011. Bird and Mammals that Depend on the Salish Sea: A Compilation. Northwestern Naturalist 92: 79-89.
Gaydos, J. K., L. Dierauf, G. Kirby, D. Brosnan, K. Gilardi and G. E. Davis. 2008. Top Ten Principles for Designing Healthy Coastal Ecosystems like the Salish Sea. EcoHealth 5: 460–471. DOI: 10.1007/s10393-009-0209-1.
Gaydos, J. K., S. Raverty, R. W. Baird, and R. W. Osborne. 2005. Suspected Surplus Killing of Harbor Seal Pups (Phoca vitulina)) by Killer Whales (Orcinus orca). Northwestern Naturalist 86(3): 150-154.
Government of Canada. 2013. Fisheries Act (R.S.C., 1985, c. F-14). http://laws- lois.justice.gc.ca/eng/acts/F-14/. Accessed 6/28/13.
Hanke, W., M. Witte, L. Miersch, M. Brede, J. Oeffner, M. Michael, F. Hanke, A. Leder, and G. Dehnhardt. 2010. Harbor Seal Vibrissa Morphology Suppresses Vortex_Induced Vibrations. The Journal of Experimental Biology 213: 2665-2672. DOI: 10.1242/jeb.043216.
Hanke, F. D., W. Hanke, C. Scholtyssek, and G. Dehnhardt. 2009. Basic Mechanisms in Pinniped Vision. Experimental Brain Research: 199: 299- 311. DOI: 10.1007/s00221-009-1793-6.
Hardee, S. E. 2008. Movements and Home Ranges of Harbor Seals (Phoca vitulina)) in the Inland Waters of the Pacific Northwest. M. S. Thesis, Western Washington University, Bellingham, Washington. 148 pp.
Harris, D. E., L. Barbara, and S. Gupta. 2003. Long-Term Observations of a Harbor Seal Haul-Out Site in a Protected Cove in Casco Bay, Gulf of Maine. Northeastern Naturalist 10(2): 141-148.
Hayes, S. A., D. E. Pearse, D. P. Costa, J. T. Harvey, B. J. Le Boeuf, and J. Carlos Garza. 2006. Mating System and Reproductive Success in Eastern Pacific Harbour Seals. Molecular Ecology 15: 3023-3034. DOI: 10.1111/j.1365-294x.2006.02984.x.
Hoover-Miller, A., A. Bishop, J. Prewitt, S. Conlon, C. Jezierski, and P. Armato. 2013. Efficacy of Voluntary Mitigation in Reducing Harbor Seal Disturbance. The Journal of Wildlife Management 77(4): 689-700. DOI: 10.1002/jwmg.510.
Hoover-Miller, A., K. R. Parker, and J. J. Burns. 2001. A Reassessment of the Impact of the Exxon Valdez Oil Spill on Harbor Seals (Phoca vitulina richardsi) in Prince William Sound, Alaska. Marine Mammal Science 17(1): 111-135.
Howard, S. M. S., M. M. Lance, S. J. Jeffries, and A. Acevedo-Gutiérrez. 2013. Fish Consumption by Harbor Seals (Phoca vitulina)) in the San Juan Islands, Washington. Fishery Bulletin 111(1): 27-41. DOI: 10.7755/FB.111.1.3.
Huber, H. R., B. R. Dickerson, S. J. Jeffries, and D. M. Lambourn. 2012. Genetic Analysis of Washington State Harbor seals (Phoca vitulina richardsi) Using Microsatellites. Canadian Journal of Zoology 90: 1361-1369. DOI: 10.1139/cjz-2012-0047.
Huber, H., and J. Laake. 2002. Trends and Status of Harbor Seals in Washington State: 1978-99. AFSC Quarterly Report October-December 2002: 1-13.
Jeffries, S., H. Huber, J. Calambokidis, and J. Laake. 2003. Trends and Status of Harbor Seals in Washington State: 1978-1999. Journal of Wildlife Management 67(1): 208-219.
Jeffries, S. J., P. J. Gearin, H. R. Huber, D. L. Saul, and D. A. Pruett. 2000. Atlas of Seal and Sea Lion Haulout Sites in Washington. Washington Department of Fish and Wildlife, Wildlife Science Division, 600 Capitol Way North, Olympia WA. 150 pp. http://wdfw.wa.gov/publications/00427/
Johnson, A., and A. Acevedo-Gutiérrez. 2007. Regulation Compliance by Vessels and Disturbance of Harbour Seals (Phoca vitulina). Canadian Journal of Zoology 85: 290-294. DOI: 10.1139/Z06-213.
Kilian, M., Dehnhardt, G. and Hanke, F.D. 2015. How harbor seals (Phoca vitulina) pursue
schooling herring. Mamm. Biol. 80: 385-389.
Lamont, M. M., J. T. Harvey, S. Jeffries, R. Brown, H. H. Huber, R. DeLong, and W. K. Thomas. 1996. Genetic Substructure of the Pacific Harbor Seal (Phoca vitulina richardsi) off Washington, Oregon, and California. Marine Mammal Science 12(3): 402-413.
Lance, M. M., W. Chang, S. J. Jeffries, S. F. Pearson, and A. Acevedo-Gutiérrez. 2012. Harbor Seal Diet in Northern Puget Sound: Implications for the Recovery of Depressed Fish Stocks. Marine Ecology Progress Series 464: 257-271. DOI: 10.3354/meps09880.
Lawson, J. W., and D. Renouf. 1985. Parturition in the Atlantic Harbor Seal, Phoca vitulina concolor. Journal of Mammology 66(2): 395-398.
Lefkovitz, L. F., V. I. Cullinan, and E. A. Crecelius. 1997. Historical Trends in the Accumulation of Chemicals in Puget Sound. NOAA Technical Memorandum NOS ORCA 111.
Lefkovitz, L. F., V. I. Cullinan, and E. A. Crecelius. 1997. Historical Trends in the Accumulation of Chemicals in Puget Sound. NOAA Technical Memorandum NOS ORCA 111.
Li, L., C. Ainsworth and T. Pitcher. 2010. Presence of Harbour Seals (Phoca vitulina) may increase exploitable fish biomass in the Strait of Georgia. Progress in Oceanography 87(1-4):235–241.
Ling, J. K. 1972. Adaptive Functions of Vertebrate Molting Cycles. American Zoologist 12(1): 77-93.
London J.M., Lance M.M., Jeffries S.J. (2001) Observations of harbor seal predation on Hood Canal salmonids from 1998 to 2000. Seattle, WA: Final Report: Studies of expanding pinniped populations NOAA Grant No. NA17FX1630, WDFW, PSMFC Contract No. 02-15. 20 p.
London, J. M., J. M. Ver Hoef, S. J. Jeffries, M. M. Lance, and P. L. Boveng. 2012. Haul-Out Behavior of Harbor Seals (Phoca vitulina) in Hood Canal, Washington. PLoS ONE 7(6): e38180. DOI: 10.1371/journal.pone.0038180.
Luxa, K., and A. Acevedo-Gutiérrez. 2013. Food Habits of Harbor Seals (Phoca vitulina) in Two Estuaries in the Central Salish Sea. Aquatic Mammals 39(1): ##-##. DOI: 10.1578/AM.39.1.2013.###.
Majewski, S. P. and Ellis, G.M. 2022. Abundance and distribution of Harbour Seals (Phoca vitulina) in the Strait of Georgia, British Columbia; synthesis of the 2014 aerial survey and long-term trends. DFO Can. Sci. Advis. Sec. Res. Doc. 2022/060. iv + 59 p.
Marston, B.H., Willson, M.F. and Gende, S.M. 2002. Predator aggregations during eulachon
Thaleichthys pacificus spawning runs. Mar. Ecol. Prog. Ser. 231: 229-236.
Mathews, E. A., and M. D. Adkison. 2010. The Role of Steller Sea Lions in a Large Population Decline of Harbor Seals. Marine Mammal Science 26(4): 803-836. DOI: 10.1111/j.1748-769.2010.00375.x.
McKnight, J.C., Bennett, K.A., Bronkhorst, M., Russell, D.J.F., Balfour, S., Milne, R., et al. 2019.
Shining new light on mammalian diving physiology using wearable near-infrared spectroscopy. PLoS Biol 17(6): e3000306. https://doi.org/10.1371/Journal.pbio.3000306
Muelbert, M. M. C., and W. D. Bowen. 1993. Duration of Lactation and Postweaning Changes in Mass and Body Composition of Harbour Seal, Phoca vitulina, Pups. Canadian Journal of Zoology 71: 1405-1414.
Nelson, B. W., C. J. Walters, A. W. Trites, and M. K. McAllister. 2023. Comparing
lethal and non‐lethal methods of active population control for harbor seals in British Columbia. Journal of Wildlife Management 87:e22400. https://doi.org/10.1002/jwmg.22400
Nelson, B. W., A. O. Shelton, J. H. Anderson, M. J. Ford, and E. J. Ward. 2019. Ecological implications of changing hatchery practices for Chinook salmon in the Salish Sea. Ecosphere 10(11):e02922. 10.1002/ecs2.2922
Nelson, B. W., Walters, C. J., Trites, A. W. & McAllister, M. K. 2018. Wild Chinook salmon productivity is negatively related to seal density and not related to hatchery releases in the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences 76, 447–462, https://doi.org/10.1139/cjfas-2017-0481.
Newby, T. C. 1973. Observations on the Breeding Behavior of the Harbor Seal in the State of Washington. Journal of Mammology 54(2): 540-543.
NOAA. 2013 (a). Marine Mammal Protection Act (MMPA). NOAA Fisheries.
http://www.nmfs.noaa.gov/pr/laws/mmpa/. Accessed 6/19/13.
NOAA. 2013 (b). Share the Shore with Harbor Seal Pup Factsheet. NOAA Fisheries Northwest Regional Office Stranding Network Resources. http://www.nwr.noaa.gov/protected_species/marine_mammals/stranding_ network_information/stranding_network_resources.html. Accessed 6/27/13.
NOAA. 2003. Harbor Seal (Phoca vitulina richardsi): Washington Inland Waters
Stock. NOAA Fisheries. http://www.nmfs.noaa.gov/pr/pdfs/sars/po2003sehr-wain.pdf. Accessed
6/19/13.
Norman, S.A.; Lambourn, D.M.; Huggins, J.L.; Gaydos, J.K.; Dubpernell, S.; Berta, S.; Olson, J.K.; Souze, V.; Evans, A.; Carlson, B.; et al. 2021. Antibiotic Resistance of Bacteria in Two Marine Mammal Species, Harbor Seals and Harbor Porpoises, Living in an Urban Marine Ecosystem, the Salish Sea, Washington State, USA. Oceans 2, 86–104. https://doi.org/10.3390/Oceans2010006
Olesiuk, P. F. 2010. An Assessment of Population Trends and Abundance of Harbour seals (Phoca vitulina) in British Columbia. DFO. Can. Sci. Advis. Sec. Res. Doc. 2009/105. vi + 157pp.
Olesiuk, P.F., M.A. Bigg, G.M. Ellis, S.J. Crockford and R.J. Wigen. 1990. An assessment of the feeding habits of Harbour Seals (Phoca vitulina) in the Strait of Georgia, British Columbia, based on scat analysis. Canadian Technical Report Fisheries and Aquatic Science No. 1730: 135p.
Olson, J.K., Lambourn, D.M., Huggins, J.L., Raverty, S., Scott, A.A., and Gaydos, J.K. 2021, Trends in Propeller Strike-Induced Mortatlity in Harbor Seals (Phoca vitulina) of the Salish Sea. Journal of Wildlife Diseases 57(3): 689-93
Olson, J. K. 2013. The Effect of Human Exposure on the Anti-Predatory Response of Harbor Seals (Phoca vitulina). M. S. Thesis, Western Washington University, Bellingham, Washington. 51 pp.
Osborne, R., J. Calambokidis, and E. M. Dorsey. 1998. A Guide to Marine Mammals of Greater Puget Sound. Anacortes, WA: Island Publishers. 191 pp.
Patterson, J., and A. Acevedo-Gutiérrez. 2008. Tidal Influence on the Haul-Out Behavior of Harbor Seals (Phoca vitulina) at a Site Available at All Tide Levels. Northwestern Naturalist 89(1): 17-23.
Peterson, S. H., M. M. Lance, S. J. Jeffries, and A. Acevedo-Gutiérrez. 2012. Long Distance Movements and Disjunct Spatial Use of Harbor Seals (Phoca vitulina) in the Inland Waters of the Pacific Northwest. PLoS ONE 7(6): e39046. DOI: 10.1371/journal.pone.0039046.
Li, L., C. Ainsworth and T. Pitcher. 2010. Presence of Harbour Seals (Phoca vitulina) may increase exploitable fish biomass in the Strait of Georgia. Progress in Oceanography 87(1-4):235–241.
Prewitt, J. S., D. V. Freistroffer, J. F. Schreer, M. O. Hammill, and J. M. Burns. 2010. Postnatal Development of Muscle Biochemistry in Nursing Harbor Seal (Phoca vitulina) Pups: Limitations to Diving Behavior? Journal of Comparative Physiology B 180: 757-766. DOI: 10.1007/s00360-010-0448-z.
Ross, P. S. 2006. Fireproof Killer Whales (Orcinus orca): Flame-retardant Chemicals and the Conversation Imperative in the Charismatic Icon of British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences 63: 224-234. DOI: 10.1139/f05-244.
Ross, P. S., M. Noël, D. Lambourn, N. Dangerfield, J. Calambokidis, and S. Jeffries. 2013. Declining Concentrations of Persistent PCBs, PBDEs, PCDEs, and PCNs in Harbor Seals (Phoca vitulina) from the Salish Sea. Progress in Oceanography (2013). DOI: 10.1016/j.pocean.2013.05.027.
Scheffer, V. B., and J. W. Slipp. 1944. The Harbor Seal in Washington State. American Midland Naturalist 32(2): 373-416.
Scheffer, T. H., and C. C. Sperry. 1931. Food Habits of the Pacific Harbor Seal, Phoca richardii. Journal of Mammology 12(3): 214-226.
Seekins, B. 2009. Harbor Seal Pupping Timeframes in Washington State. NOAA Fisheries Northwest Regional Office. http://www.nwr.noaa.gov/maps_data/marine_mammal_maps.html.
Retrieved online 6/12/13.
Shields, M.W., Hysong-Shimazu, S., Shields, J.C., and Woodruff. J. 2018. Increased presence of mammal-eating killer whales in the Salish Sea with implications for predator-prey dynamics.
Simpkins, M. A., D. E. Withrow, J. C. Cesarone, and P. L. Boveng. 2003. Stability in the Proportion of Harbor Seals Hauled Out Under Locally Ideal Conditions. Marine Mammal Science 19(4): 791-805.
Smith, T. G., M. O. Hammill, and G. Taugbøl. 1990. A Review of the Developmental, Behavioural and Physiological Adaptations of the Ringed Seal, Phoca hispida, to Life in the Arctic Winter. Arctic 44(2): 124-131.
Stein, J. 1989. Reproductive Parameters and Behaviors of Mother and Pup Harbor Seals, Phoca vitulina richardsi, in Grays Harbor, Washington. M. S. Thesis, San Francisco State University, San Francisco, California. 110 pp.
Stutz, S. S. 1967. Moult in the Pacific Harbour Seal Phoca vitulina richardi. Journal of the Fisheries Research Board of Canada 24(2): 435-441.
Sullivan, R. M. 1981. Aquatic Displays and Interactions in Harbor Seals, Phoca vitulina, with Comments on Mating Systems. Journal of Mammalogy 62(4): 825-831.
Suryan, R. M., and J. T. Harvey. 1999. Variability in Reactions of Pacific Harbor Seals, Phoca vitulina richardsii, to Disturbance. Fishery Bulletin 97(2): 332-339.
Suryan, R. M., and J. T. Harvey. 1998. Tracking Harbor Seals (Phoca vitulina richardsi) to Determine Dive Behavior, Foraging Activity, and Haul-out Site Use. Marine Mammal Science 14(2): 361-372.
Tabuchi, M., N. Veldhoen, N. Dangerfield, S. Jeffries, C. C. Helbing, and P. S. Ross. 2006. PCB-Related Alteration of Thyroid Hormones and Thyroid Hormone Receptor Gene Expression in Free-Ranging Harbor Seals (Phoca vitulina). Environmental Health Perspectives 114(7): 1024-1031.
Taggart, S. J., A. G. Andrews, J. Mondragon, and E. A. Mathews. 2005. Co- occurrence of Pacific Sleeper Sharks Somniosus pacificus and Harbor Seals Phoca vitulina in Glacier Bay. Alaska Fishery Research Bulletin 11(2): 113-117.
Tallman, J., and C. Sullivan. 2004. Harbor Seal (Phoca vitulina) Predation on a Male Harlequin Duck (Histrionicus histrionicus). Northwest Naturalist 85: 31-31.
Temte, J. L. 1985. Photoperiod and the Timing of Pupping in the Pacific Harbor Seal (Phoca vitulina richardsi) with Notes on Reproduction in Northern Fur Seals and Dall Porpoises. M. S. Thesis, Oregon State University, Corvallis, Oregon. 147 pp.
Thomas, A.C., Deagle, B., Nordstrom, C., Majewski, S., Nelson, B.W., Acevedo-Gutierrez, A., Jeffries, S., Moore, J., Louden, A., Allegue, H., Pearson, S., Schmidt, M., Trites, A.W. 2022. Data on the diets of Salish Sea harbour seals from DNA metabarcoding. Scientific data 9(68). https://doi.org/10.1038/s41597-022-01152-5
Thomas, A. C., M. M. Lance, S. J. Jeffries, B. G. Miner, and A. Acevedo- Gutiérrez. 2011. Harbor Seal Foraging Response to a Seasonal ResourcePulse, Spawning Pacific Herring. Marine Ecology Progress Series 441: 225-239. DOI: 10.3354/mesp09370.
Thomas, A. C., Nelson, B. W., Lance, M. M., Deagle, B. E. & Trites, A. W. 2017. Harbour seals target juvenile salmon of conservation concern. Canadian Journal of Fisheries and Aquatic Sciences 74, 907–921, https://doi.org/10.1139/cjfas-2015-0558.
Thompson, P. M., H. M. Corpe, and R. J. Reid. 1998. Prevalence and Intensity of the Ectoparasite Echinophthirius horridus on Harbour Seals (Phoca vitulina): Effects of Host Age and Inter-annual Variability in Host Food Availability. Parasitology 117(4): 393-403.
Thompson, D. M., D. Miller, R. Cooper, and P. S. Hammond. 1994. Changes in the Distribution and Activity of Female Harbour Seals During the Breeding Season: Implications for their Lactation Strategy and Mating Patterns. Journal of Animal Ecology 63(1): 24-30.
Vancouver Aquarium. 2013. Marine Mammal Rescue. https://www.vanaqua.org/marine-mammal-rescue/. Accessed 8/8/23.
van Parijs, S.M., Thompson, P.M., Tollit, D.J. and Mackay, A. 1997. Distribution and activity
of male harbour seals during the mating season. Anim. Behav. 54: 35-43.
Walker, E. P., and R. M. Nowak. 1999. Walker’s Mammals of the World, 6th Ed. The Johns Hopkins University Press. Baltimore, Maryland. 1936 pp.
Ward, E. J., P. S. Levin, M. M. Lance, S. J. Jeffries, and A. Acevedo-Gutiérrez. 2012. Integrating Diet and Movement Data to Identify Hot Spots of Predation Risk and Areas of Conservation Concern for Endangered Species. Conservation Letters 5: 37–47.
Washington State Academy of Sciences. (2022). Pinniped Predation on Salmonids in
the Washington Portions of the Salish Sea and Outer Coast. Seattle, WA: WSAS, 1-81
Watts, P. 1996. The Diel Hauling-Out Cycle of Harbour Seals in an Open Marine Environment: Correlates and Constraints. Journal of Zoology 240: 175- 200.
Wilson, K. M. Lance, S. Jeffries, and A. Acevedo-Gutiérrez. 2014. Fine-Scale Variability in Harbor Seal Foraging Behavior. PLOS One 9(4): e92838. doi:10.1371/journal.pone.0092838
Zamon, J. 2001. Seal Predation on Salmon and Forage Fish Schools as a Function of Tidal Currents in the San Juan Islands, Washington, USA. Fisheries Oceanography 10(4): 353-366.
Zier, J.C. and Gaydos, J.K. 2014. Harbor seal species profile. Encyclopedia of Puget Sound.
Licensing & attribution
Data and products from the PSEMP Marine Mammal Work Group are governed by a Creative Commons BY-NC-SA license. Attribution should be to: “PSEMP Marine Mammal Work Group” with a link to https://psemp.net/mmwg







![Map showing harbor seal pupping time frames by location in the Salish Sea.]](https://www.eopugetsound.org/sites/default/files/styles/article_inline_slideshow/public/topical_articles/images/PuppingTimeframeWA_1024.jpg?itok=sByZzYqw)


