Status and trends of harbor porpoises in the Salish Sea
Harbor porpoises declined dramatically in the Salish Sea in the 1970s but their populations have since rebounded, increasing by more than 10% per year in recent decades. A 2020 report for the Encyclopedia of Puget Sound examines harbor porpoise status and trends, natural history and recent policy considerations for the species.
Overview
Harbor porpoises are the smallest cetaceans in the Salish Sea, but an important part of the ecosystem as both predator and prey. They feed on smaller fish species, such as herring, pollock, and hake (Nichol et al. 2013), although they have been observed on occasion to catch (consumption was not observed) salmonid species (Elliser et al. in press). Their only natural predator is the Bigg’s killer whale, which is increasingly active within the Salish Sea in recent years.
While harbor porpoises were common in Puget Sound in the 1940s (Scheffer and Slipp 1948), their numbers were greatly reduced throughout Washington’s inland waters in the late 20th century. The population has been recovering since the mid 1990s and today they are found throughout the Salish Sea year-round. Population densities are rapidly rising in Puget Sound and appear to have reached carrying capacity in the San Juan Islands around the year 2000 (Evenson et al. 2016)
Harbor porpoises have regular site fidelity in certain areas (Elliser et al. 2018), and spend the majority of their time in nearshore habitats. Thus, they are sensitive to human activities, including key threats such as noise pollution, habitat degradation, loss of prey, and interaction with fishing gear (especially gill nets). Information is limited on this cryptic species and more research is needed to be able to determine best protective management recommendations.
Trends and Events
Harbor porpoises were abundant in the Salish Sea into the 1940s (Scheffer and Slipp 1948) but declined dramatically by the 1970s (Evenson et al. 2016, Osmek et al. 1996, Raum-Suryan and Harvey, 1998). This decline was particularly evident in the Puget Sound, where harbor porpoises were virtually absent (see Evenson et al. 2016). Since the mid 1990s harbor porpoises numbers have increased, and after about 2 decades of growth in the inner marine waters of Washington State at an annual rate of 10.4% (1995-2014), they have been observed in all U.S. areas of the Salish Sea (Evenson et al. 2016, Jefferson et al. 2016). The reasons for their decline and recovery are not fully understood, but are likely related to fisheries interactions, pollution and habitat loss/degradation. As these negative influences have been better managed and reduced, harbor porpoises have returned to their traditional habitats (Jefferson et al. 2016).
Populations in Washington Sound (including San Juan Islands and the Strait of Georgia), Strait of Juan de Fuca, and Puget Sound (Admiralty Inlet, Hood Canal, Whidbey Basin, Central Puget Sound and South Puget Sound) showed varying increases in density from aerial surveys 1995-2015 (Evenson et al. 2016).
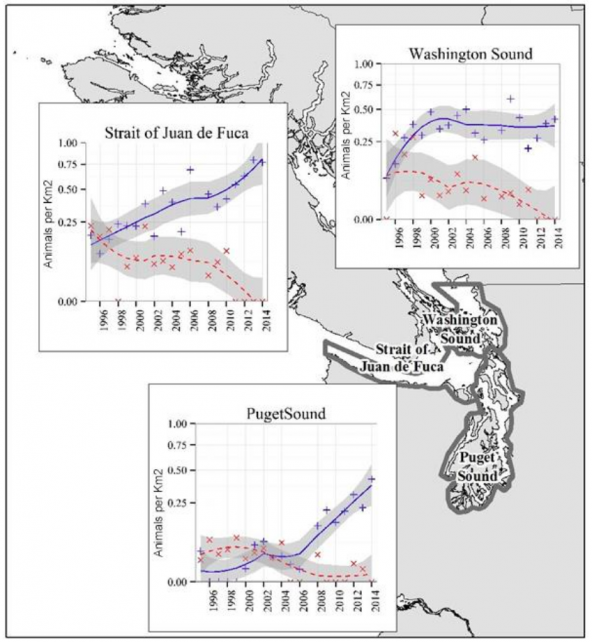
The elevation of observed growth rates well above maximum theoretical reproductive growth rates indicates that there was immigration of animals from outside the study area, either inland British Columbia waters or coastal regions of Washington and/or British Columbia (Evenson et al. 2016). While the abundance of harbor porpoises in Washington Sound leveled off, incursions into Puget Sound increased, suggesting that the Puget Sound animals came from the Strait of Juan de Fuca, Puget Sound, Canadian and/or coastal waters, and may be a consequence of resource limitation in these northeastern areas (Evenson et al. 2016).
The most recent abundance estimate for the Washington Inland Waters stock, during spring is 11,233 porpoises (density 1.58 porpoises/km2, CV=37%, 95% confidence intervals are 9,616-13,120) (Jefferson et al. 2016). Evidence for this increase in population has also been documented in the stranding record. Analysis of an unusual mortality event (UME) in 2006-2007, and monitoring for four years after the event, revealed that the increased strandings of harbor porpoises in Washington and Oregon were attributed to an increase in their population coupled with improved reporting (Huggins et al. 2015). The two most commonly diagnosed conditions for primary and/or secondary cause of death were infectious disease (various bacteria, viruses, protozoans and fungus) and trauma (including asphyxiation, interspecific trauma, subcutaneous hemorrhaging, and fishery related interactions) (Huggins et al. 2015). One disease of concern is Cryptococcus gattii, a yeast that can be transmitted between a female porpoise and a fetus (Norman et al. 2011). Several novel fungal pathogens are of concern. Cryptococcus gattii has be documented in multiple harbor porpoise (citation) from point sources since 2001 (Stephen 2002) and has been shown to be transmitted transplacentally (Norman et al. 2011). Mucormycosis has been recognized as a cause of mortality more recently.
Interestingly, the harbor porpoise population increase coincides with marked decreases in Dall’s porpoise abundance at all locations in Washington (Evenson et al. 2016). There is significant overlap in the diet of Dall’s and harbor porpoises, although there is some evidence for resource and habitat partitioning (discussed further below) (Hall 2011, Nichol et al. 2013). Interspecific mating between male harbor porpoises and female Dall’s porpoises has occurred (Willis et al. 2004) and may be disruptive to Dall’s porpoises (Jefferson et al. 2016). Papers published before the 1960s, when harbor porpoises were abundant, suggest that Dall’s porpoises were rarely seen during that time (Evenson et al 2016). Thus the abundance of these two closely related species may be inversely correlated due to ecological and behavioral interactions.
Natural history
Distribution and occurrence
Harbor porpoises are found throughout the Canadian and US waters of the Salish Sea, usually found within a few miles of shore. They are often found in areas with large tidal flows that pass through narrow straits or around headlands, known as tidal-stream habitats (Benjamins et al. 2016) and may contain hydrological components such as eddies, boils and fronts that provide predictable, favorable foraging conditions, such as enhanced prey abundance, vulnerability and/or diversity (Benjamins et al. 2015) and thus are ideal for harbor porpoises that need consistent, predictable food sources.
Sightings occur in every season for most areas of the Salish Sea. In aerial surveys Jefferson et al. (2016) found that the San Juan Islands area contained the highest densities of harbor porpoises. Harbor porpoise density (porpoises/km2) of the different regions, from highest to lowest: San Juan Island- 2.16, North Puget Sound- 1.54, Hood Canal- 0.73, Strait of Juan de Fuca (limited survey effort)- 0.71, and South Puget Sound- 0.68.
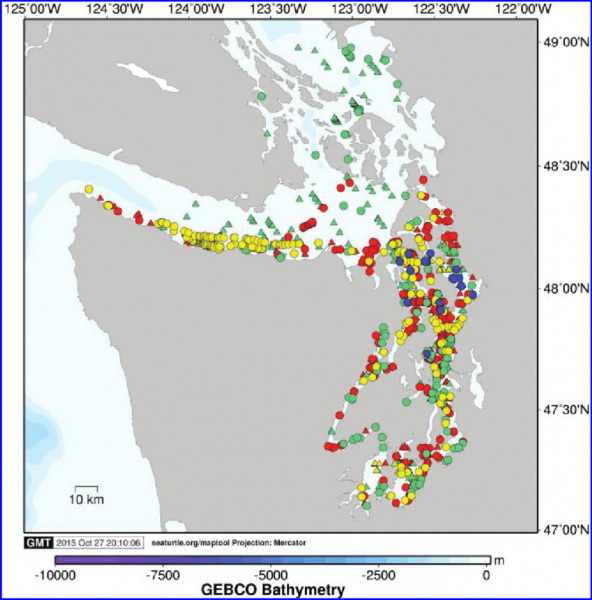
Map: Jefferson, Thomas et al. (2016). Harbor porpoise (Phocoena phocoena) recovery in the inland waters of Washington: Estimates of density and abundance from aerial surveys, 2013-2015. Canadian Journal of Zoology. 94. 10.1139/cjz-2015-0236. ©Canadian Science Publishing or its licensors.
Genetic analysis has revealed that this Washington Inland Waters stock is non-migratory and genetically distinct from the outer coast stocks, further supporting that harbor porpoises use the Salish Sea year-round (Carretta et al. 2018). However, Jefferson et al. (2016) and Evenson et al. (2016) noted that the Washington Inland Waters stock is not limited to Washington inland waters and movement into Canadian waters and west into the outer coastal waters are likely.
Despite this, there is evidence that harbor porpoises may have relatively small home ranges and movement patterns. Limited data from pilot photo-identification studies and VHF tagged animals in the Salish Sea indicate geographic fidelity and relatively small home ranges (Flaherty and Stark 1982, in Baird 2003; Hanson 2007). Recent long-term research using photo-identification supports these findings and has revealed that some harbor porpoises continually return (across weeks, seasons and years) to waters off Fidalgo Island, WA and thus may have stronger site fidelity to certain areas than previously understood (Elliser et al. 2018). Further research is required to better understand the site fidelity, population structure and movement patterns of harbor porpoises.
What eats them?
The only known natural predator for harbor porpoises in Puget Sound is the Bigg’s killer whale, which is increasing in abundance in recent years (Shields et al. 2018). Observations have shown that all regularly encountered groups of Bigg’s killer whales predate upon both pinnipeds and cetaceans (Ford et al. 1998).
In other areas, including off the outer coast of British Columbia, great white sharks predate on harbor porpoises. Although there are not any official documented encounters with great white sharks in the Salish Sea, they are common along the coast of Washington and could come into the area occasionally.
Members of the southern resident killer whales, including L pod and more recently J pod, are sometimes observed to kill but not consume harbor porpoises (see this synopsis in the Encyclopedia of Puget Sound).
What do they eat?
Globally harbor porpoises have a non-specialized diet including a variety of fish and invertebrates, often including large amounts of small forage fish. In the Salish Sea, stomach contents of stranded harbor porpoises (primarily from the Strait of Juan de Fuca) indicated 12 different prey species in 1991-2010 (Nichol et al. 2013) and 17 different prey species in 1990-1997 (Walker et al. 1998). There were five species that were found in both studies: Pacific herring, Walleye pollock, Pacific hake, Pacific sand lance, and Polychaete species (likely secondary prey species eaten first by the fish prey). There is significant overlap in the diet of Dall’s and harbor porpoises, including Pacific herring (most important for both species), Walleye Pollock and Pacific hake; but there is some evidence for resource and habitat partitioning, which may vary seasonally and spatially (Nichol et al. 2013).
These studies reveal that Pacific herring, Walleye pollock and Pacific hake are the most important prey items for harbor porpoises as they topped the lists for both amount ingested and percentage of caloric intake. However, there are obvious variations in prey species documented in each study. For example Blackbelly eelpout was significant in Walker et al. (1998), but not found in Nichol et al. (2013), and conversely Blackfin sculpin was significant in Nichol et al. (2013), but not found in Walker et al. (1998). Relative abundance of prey species in the diets of harbor porpoises may vary between locations, years and season (Nichol et al. 2013) and may be related to local prey abundance.
Recently it has been documented that harbor porpoises sometimes target larger fish (more so than commonly known prey species), including salmonids. Harbor porpoises were observed chasing and catching salmonid species off Fidalgo Island, Washington, although consumption of the fish was not observed (Elliser et al. in press). Similar events involving American shad were documented in San Francisco Bay, CA, along with a case in Cook Inlet Alaska where a female harbor porpoise drowned in a drift net with salmon in her stomach and throat (Elliser et al. in press). For harbor porpoises, metabolizable energy intake estimates are most strongly affected by variations in target prey size (followed by foraging intensity); thus consideration of prey species, target size and energy content are critical in assessing how a species exists in its ecological niche (Booth, 2019). It is evident that harbor porpoises sometimes target larger prey items. Further research is needed to fully understand their behavioral repertoire and ecological relationships.
Biology
Harbor porpoises are the second smallest cetacean in the world and live in relatively cold waters. They have high metabolic rates (Kanwisher and Ridgeway 1983; Gallagher et al., 2018; Rojano-Doñate et al., 2018) and, due to their small size, are limited in the amount of energy they can store (MacLeod et al. 2007). Thus harbor porpoises can only survive short periods without food (as little as three days, MacLeod et al. 2007) making finding consistent prey sources critical for their survival. This may be more important for females because if they are breeding every year, they will be simultaneously lactating and pregnant (Read and Hohn 1995; Norman et al. 2018); which creates a greater energetic incentive to find consistent areas where high caloric prey species are abundant (Gilles et al. 2009) and/or select larger than average length prey (Yasuiw and Gaskin 1986).
Harbor porpoises breed every one or two years, depending on location. Reproductive timing is less known for Salish Sea harbor porpoises, but a recent study revealed stranded females that were both pregnant and lactating, indicating that they can breed every year (Norman et al. 2018). Conception likely occurs between August and December, with a gestation averaging 10.8 months and calving occurring between July to November (Norman et al. 2018). This is supported by other research documenting calf sightings from May to September, often peaking in later summer/early fall in Washington waters (Raum-Suryan and Harvey 1998; Elliser et al. 2018) and Canadian waters (Hall 2011). Specific areas important for mating and reproduction are not currently known for this population in Washington waters, however possible breeding sites have been identified in Canadian waters (Hall 2011).
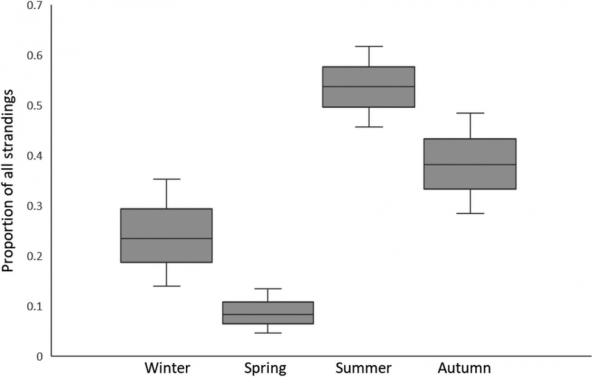
Graph: Norman, Stephanie et al. (2018). Conception, fetal growth, and calving seasonality of harbor porpoise (Phocoena phocoena) in the Salish Sea waters of Washington State, USA and southern British Columbia, Canada. Canadian Journal of Zoology. 96. 10.1139/cjz-2017-0155. ©Canadian Science Publishing or its licensors.
Harbor porpoises are thought to ‘live life in the fast lane’ as they have relatively shorter life spans compared to other marine mammals. Although there is little data on the life-span for the Salish Sea population, studies of stranded individuals in other populations show that although harbor porpoises can reach over 20 years of age, the vast majority do not live past 10 years (Read and Hohn 1995). Due to this quick time frame, males and females become sexually mature earlier around four years of age (Hohn & Brownell, 1990). Support for the likely shorter life span for harbor porpoises is found in their physiological development as well. In the Salish Sea, harbor porpoises show muscle maturation at an earlier age than what has been found previously for dolphin species (Noren et al. 2014).
Behavior
Harbor porpoises are also known as ‘puffing pigs’ due to the often disproportionate loud sound of their exhale compared to their small physical size. This species can be shy and evasive, generally doesn’t approach boats and often surfaces in erratic patterns making it difficult to observe. Information on their behaviors is thus limited, but recent research is shedding light on this elusive species.
Harbor porpoises in the Salish Sea (as in other areas around the world) differ from most dolphin species in that they are generally seen in small groups of less than three animals (Raum-Suryan and Harvey 1998, Jefferson et al. 2016, Elliser et al. 2018) and it is not uncommon to see them solitary or in pairs. There have been reports of large aggregations of over 50 or 100 individuals in areas around the Salish Sea, though they are not common. Although it is unclear why these aggregations occur, social and surface-active behaviors often increase as porpoise densities increase (Hall 2011). This may indicate that harbor porpoises have more developed social behaviors than is currently understood (Hall 2011).
Although there is limited information about the sociality of this species, some recent work supports the idea that this cryptic species is more social than previously thought. Research in San Francisco has revealed insights into harbor porpoise mating behaviors, noting that many aerials seen may actually be mating attempts and that they are stereotyped where the male always approaches the female on the left side (Keener et al. 2018). These behaviors have also been documented photographically in the Salish Sea by Pacific Mammal Research (see figure, C. Elliser personal observation). In both locations they are seen in all seasons.

Though less common than in other species, harbor porpoises will surf in waves and boat wakes (see figure, C. Elliser personal observation), often once the boat has passed (i.e. they are not actively following the boat in the wake). These types of behaviors increase during high-density social events (Hall 2011).

Harbor porpoises are often described as acoustically cryptic and less social, as they vocalize using narrow band high frequency (NBHF) clicks and don’t seem to have whistles (which are commonly used in dolphins for communication). Recent evidence, however, suggests that they used these NBHF clicks for social interactions that may be more important than the limited social interactions observed at the surface may indicate (Sorensen et al. 2018). More research is needed to better understand the behaviors and social structure of this species.
Data related to current policy issues
Harbor porpoises and harbor seals are main prey species for Bigg’s killer whales, which are increasing in numbers in recent years (Shields et al. 2018). Harbor porpoise populations could be highly impacted if proposed predator culling of harbor seals occurs in an effort to reduce predation on salmon populations. This would effectively reduce the amount of food available for the Bigg’s killer whales and possibly force them to increase their take of harbor porpoises.
Harbor porpoises are also an important predator in this ecosystem, helping to maintain prey populations and ecosystem balance. Removal of top predators can greatly alter and damage an ecosystem, and thus it is critical to understand more about harbor porpoises in the Salish Sea in order to make informed decisions on the management of this unique ecosystem.
Data sources and gaps
Until recently there has been little focused monitoring of harbor porpoises in the Salish Sea. Previous data come from stranding records, various short-term studies and periodic aerial surveys, some of which were dedicated surveys for harbor porpoises (e.g. Jefferson et al. 2016), whereas others were designed for more general marine life (e.g. Evenson et al. 2016). However, interest in harbor porpoises is increasing. Lack of information about this species has been identified as a data gap, and the need for monitoring has been recognized. Long-term monitoring studies are being conducted by collaborating groups around the Salish Sea (e.g. Pacific Mammal Research (Elliser et al. 2018, in press), Porpoise Conservation Society, Cascadia Research Collective).
Data from any continued marine aerial surveys (Evenson et al. 2016), increased collaboration with monitoring efforts in Canada, and long-term monitoring are be crucial to better understand the ecology and behavior of this species and to better manage and conserve it in the Salish Sea.
References
Baird, R. W. (2003). Update COSEWIC status report on the harbour porpoise Phocoena phocoena
(Pacific Ocean population) in Canada, in COSEWIC assessment and update status report on the harbour porpoise Phocoena phocoena (Pacific Ocean population) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, Canada. 22 pp.
Benjamins, S., Dale, A., Hastie, G., Waggitt, J. J., Lea, M.-A., Scott, B. E., & Wilson B. (2015). Confusion reigns? A review of marine megafauna interactions with tidal-stream environments. Oceanography and Marine Biology: An Annual Review 53:1–54.
Benjamins, S., Dale, A., van Geel, N. & Wilson, B. (2016). Riding the tide: Use of a moving tidal-stream habitat by harbour porpoises. Marine Ecology Progress Series 549:275–288.
Booth, C. G. (2019). Food for thought: Harbor porpoise foraging behavior and diet inform vulnerability to disturbance. Marine Mammal Science, doi:10.1111/mma.12632.
Carretta, J.V., Forney, K.A., Oleson, E.M., et al. (2019) U.S. Pacific Marine Mammal Stock Assessments: 2018. U.S. Department of Commerce, NOAA Technical Memorandum NMFSSWFSC-617, 382pp.
Elliser, C. R., MacIver, K. H. & Green, M. (2018). Group characteristics, site fidelity, and photo-identification of harbor porpoises, Phocoena phocoena, in Burrows Pass, Fidalgo Island, Washington. Marine Mammal Science 34(2), 365–384.
Elliser, C. R., Hessing, S., MacIver, K. H., Webber, M.A., Keener, W. (in press). Harbor porpoises (Phocoena phocoena vomerina) catching and handling large fish on the U.S. West Coast. Aquatic Mammals.
Evenson, J. R., Anderson, D., Murphie, B.L., Cyra, T.A., & Calambokidis, J. (2016). Disappearance and Return of Harbor Porpoise to Puget Sound: 20 Year Pattern Revealed from Winter Aerial Surveys. Technical Report. Washington Department of Fish and Wildlife, Wildlife Program and Cascadia Research Collective, Olympia, WA. 26pp.
Ford, J. K., Ellis, G. M., Barrett-Lennard, L. G., Morton, A. B. , Palm, R. S., & Balcomb, K. C. B. (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Canadian Journal of Zoology 76:14561471 DOI 10.1139/z98-089.
Gallagher, C. A., Stern, S. J., & Hines, E. (2018). The metabolic cost of swimming and reproduction in harbor porpoises (Phocoena phocoena) as predicted by a bioenergetic model. Marine Mammal Science, 34(4), 875-900. doi: 10.1111/mms.12487
Gilles, A., Scheidat, M., & Siebert, U. (2009). Seasonal distribution of harbour porpoises and possible interference of offshore wind farms in the German North Sea. Marine Ecology Progress Series 383:295–307.
Hall, A. 2011. Foraging behaviour and reproductive season habitat selection of northeast Pacific porpoises. Ph.D. thesis, University of British Columbia, Vancouver, Canada. 197 pp. Retrieved from http://www.marinemammal.org/wp-content/pdfs/Hall%202011.pdf.
Hanson, M. B. (2007). Seasonal movements and habitat use of Dall’s and harbor porpoises in the inland and coastal waters of Washington State as determined by radiotelemetry. Report of the National Marine Fisheries Service workshop on advancing electronic tag technology and their use in stock assessments. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-F/SPO-82 (pp 53-54). Eds. P. Sheridan, J.W. Ferguson and S.L. Downing.
Hohn, A. A., & Brownell Jr., R. L. (1990). Harbor porpoise in central Californian waters: life history and incidental catches. Paper SC/42/SM47 presented at 42nd Meeting of the Scientific Committee, International Whaling Commission, Nordwijk, Holland.
Huggins, J.L., Raverty, S.A., Norman, S.A., et al. (2015). Increased harbor porpoise mortality in the Pacific Northwest, USA: understanding when higher levels may be normal. Diseases of Aquatic Organisms, 115, 93-102.
Jefferson, T. A., Smultea, M. A., Courbis, S. S., & Campbell, G. S. (2016). Harbor porpoise (Phocoena phocoena) recovery in the inland waters of Washington: estimates of density and abundance from aerial surveys, 2013–2015. Canadian Journal of Zoology, 94, 505–515.
Kanwisher, J. W., & Ridgeway, S. H. (1983). The physiological ecology of whales and porpoises. Scientific American 248(6):110–120.
Keener, W., Webber, M. A., Szczepaniak, I. D., Markowitz, T. M., & Orbach, D. N. (2018). The sex life of harbor porpoises (Phocoena phocoena): lateralized and aerial behavior. Aquatic Mammals, 44(6), 620-632.
MacLeod, C. D., Santos, M. B., Reid, R. J., Scott, B. E., & Pierce, G. J. (2007). Linking sandeel consumption and the likelihood of starvation in harbour porpoises in the Scottish North Sea: Could climate change mean more starving porpoises? Biology Letters, 3, 185–188. doi: 10.1098/rsbl.2006.0588
Nichol, L. M., Hall, A. M., Ellis, G. M., Stredulinsky, E., Boogaards, M. & Ford, J. K. B. (2013). Dietary overlap and niche partitioning of sympatric harbour porpoises and Dall’s porpoises in the Salish Sea. Progress in Oceanography, 115, 202–210.
Noren, S. R., Noren D. P., & Gaydos, J. K. (2014). Living in the fast lane: Rapid development of the locomotor muscle in immature harbor porpoises (Phocoena phocoena). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 184, 1065–1076.
Norman, S. A., Raverty, S., Zabek, E., Etheridge, S., Ford, J. K. B., Hoang, L. M. N., & Morshed, M. Maternal-fetal transmission of Cryptococcus gattii in harbor porpoise. Emerging Infectious Diseases, 17(2), 304-305.
Norman, S. A., Hanson, M. B., Huggins, J. et al. (2018). Conception, fetal growth, and calving seasonality of harbor porpoise (Phocoena phocoena) in the Salish Sea waters of Washington, USA, and southern British Columbia, Canada. Canadian Journal of Zoology, 96, 566-575.
Osmek, S., Calambokidis, J., Laake, J. et al. (1996). Assessment of the status of harbor porpoise
(Phocoena phocoena) in Oregon and Washington waters. U.S. Department of Commerce,
NOAA Technical Memorandum, NMFS-AFSC-76, 46 pp.
Raum-Suryan, K. L. & Harvey, J. T. (1998). Distribution and abundance of and habitat use by harbor porpoise, Phocoena phocoena, off the northern San Juan Islands, Washington. Fishery Bulletin, 96, 808–822
Read, A. J., & Hohn, A.A., (1995). Life in the fast lane: The life history of harbor porpoises from the Gulf of Maine. Marine Mammal Science, 11, 423–440.
Rojano-Doñate, L., McDonald, B.I., Wisniewska, D.M., et al. (2018). High field metabolic rates of wild harbour porpoises. Journal of Experimental Biology, 221(23), jeb185827. doi: 10.1242/jeb.185827
Scheffer, V. B., & Slipp, J. W. (1948). The Whales and Dolphins of Washington State with a Key to the Cetaceans of the West Coast of North America. The American Midland Naturalist 39, 257–337.
Shields, M. W., Hysong-Shimazu, S., Shields, J. C., & Woodruff, J. (2018). “Increased presence of mammal-eating killer whales in the Salish Sea with implications for predator-prey dynamics”. PeerJ 6: e6062. DOI 10.7717/peerj.6062
Sorensen, P. M., Wisniewska, D. M., Jensen, F. H., Johnson, M., Teilmann, J. & Madsen, P. T. (2018). Click communication in wild harbour porpoises (Phocoena phocoena). Scientific Reports 8,9702. DOI:10.1038/s41598-018-28022-8
Walker, W. A., Hanson, M. B., Baird R. W., & Guenther, T. J. (1998). Food habits of the harbor porpoise, Phocoena phocoena, and Dall’s porpoise, Phocoenoides dalli, in the inland waters of British Columbia and Washington. Alaska Fisheries Science Center Processed Report, 98(10), 63–75.
Willis, P. M., Crespi, B. J., Dill, L. M., Baird, R. W., & Hanson, M. B. (2004). Natural hybridization between Dall’s porpoises (Phocoenoides dalli) and harbour porpoises (Phocoena phocoena). Candian Journal of Zoology, 94(7), 505-515.
Yasuiw, Y. & Gaskin, D.E. (1986). Energy budget of a small cetacean, the harbour porpoise, Phocoena phocoena. Ophelia, 25, 183-197.






